Describe Atomic Number in Your Own Words
Describe each of the sub atomic particles protons neutrons and electrons. For each type explain how the atomic number and the atomic mass change.
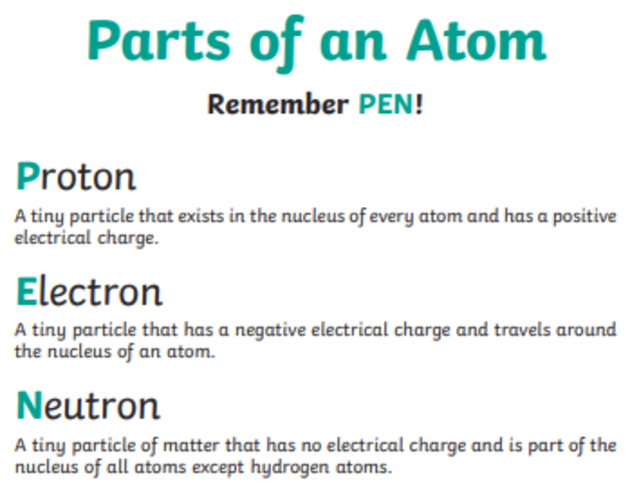
What Is Atomic Structure Definition Meaning And Resources
Beta decay occurs when the nucleus of an atom has too many neutrons.
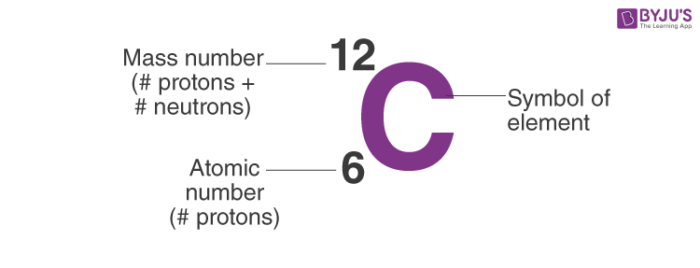
. The order of an element in Mendeleyevs table of the elements. You need to use the neutron gun to. Define atomic number in your own words.
Be sure to include. For each type describe how the atomic number and atomic mass change. In completely occupied atomic orbitals ie.
Looking at your arrangement of the element cards describe in your own words what the term periodic function means. Write balanced chemical reactions for the following. Define and provide an example for an element compound and mixture.
State the principle of fossil succession in your own words. 3element with different atoms can chemically combined in simple whole number ratios. In your own words describe what is occurring during the process of electron beta decay explaining what is ejected from the unstable nucleus and focusing subatomic changes.
621 C 521 H 121 N and the remainder Oxygen. - 2207821 Hala18 Hala18 11132016 Chemistry High School answered In your own words explain the. Gallium-66 undergoes electron capture 60 27 Co 60 28 Ni 0 1 electron 241 95 Am 4 2 He 237 93 Np 60 29 Cu 0 1 electron 60 28 Ni.
They have mass numbers of 28 29 and 30The atomic mass of silicon is 28086 amu. In simple terms an atom is a cloud of tiny electrons buzzing round a central much larger nucleus in a series of orbits called shells. Be able to define each step of the scientific method.
Comment on the relative abundance of these three isotopes. List three ways that unstable nuclei change. Start your trial now.
If the number of protons in an atom is 13 and the number of neutrons is 14 i what is the atomic number of the atom. Draw a food chain involving snake at one of its level. Describe what you would do to make U-235 unstable both in terms of what you see and do in the simulation and what this represents physically.
List three ways that unstable nuclei change. In your own words describe what is occurring during the process of electron beta decay. Total number of electrons in an element Atomic number of that element.
In your own words state the main ideas of Daltons atomic theory. Equal to the number of protons in the nucleus or electrons in the neutral state of an atom of an element. In your own words clearly describe the structure of the atomas we currently understand it.
Use an example of an element if it you with your description. The beta particles are high in energy electrons so during the electron-beta decay from an unstable nucleus a neutron. 1 point each 4 points total Cobolt-60 undergoes beta decay.
Some of the the properties listed on each card are periodic properties others are not. The magnetic quantum number denoted by the symbol m l Furthermore it can be noted that each atomic orbital can hold a maximum of two electrons. Be able to define each step of the scientific method.
You have an atom of carbon with a mass number of 14. In your own words using your own example explain the scientific method. Alpha emission - atomic number -2 atomic mass-4 Beta emission - atomic number 1 atomic mass-no change.
Match the description of each organism to the appropriate category. Consider what you learned from this simulation. Describe each of the sub atomic particles protons neutrons and electrons.
There are three isotopes of silicon. Carbon has atomic number six this tells that carbon atom has six protons present in it and six elect. The nucleus emits a beta particle electron that is charged and the atomic number is increased by 1 but the atomic mass remains the same.
How do living things obtain phosphorus. The ratio of neutrons to protons is too high. The other 2 isotopes must be present in very small amounts.
Protons- 6 Neutrons- 8 Electrons- 6 4. The locations of these particles within the atom. Reflect on three to four key concepts that you learned in this lab exercise.
To write the electronic configuration first count the total number of electrons in an element. Silicon-28 must be by far the most abundant. 2atoms of the same element are the same while atoms of different elements are different.
The atomic orbitals containing two electrons each of the electrons has an equal and opposite spin when compared to the other. For filling of electrons we follow some rules. In your own words explain the main ideas of Daltons atomic theory.
In your own words using your own example explain the scientific method. Solution for In your own words describe the operation of a clinical Laboratory. Cobolt-60 undergoes beta decay.
The orbitals with lowest energy will be filled first. Describe how the atomic number and atomic mass of an element are calculated. Determine the number of protons neutrons and electrons you would expect this atom to possess.
2 points for clear and accurate description. 2 points for clear and accurate description. Describe food chain.
In your own words what does unstable mean when used to describe Uranium. Strontium-89 is an isotope used in the treatment of bone cancer and has a half-life of 5057 days. Know the difference between a dependent and independent variable.
Know the difference between a dependent and independent variable. In youre own words no guessing40 points science in your own words explain what the atomic number and atomic mass of an element represent. The mass composition of a compound is.
In your own words describe what is occurring during the process of electron beta decay explaining what is ejected from the unstable nucleus and focusing subatomic changes. State the principle of fossil succession in your own words. Name one property that is periodic and one that is not.
First week only 499. 66 31 Ga 0 1 electron 66 30 Zn 3. Up to 24 cash back ments are periodic functions of their atomic masses.
Atomic number of Nickel 28. In your own words explain the periodic law. 1all elements are made up of atoms.
The relative masses and charges of the three principle subatomic particles and ii. 1 Show answers Another question on Biology. So number of electrons 28.
Define and provide an example for an element compound and a mixture. Definitions of atomic number. Atoms are very very small and make up everything in the natural world According to Daltons theory is it possible to convert atoms of one element into atoms of anotherExplain.

Atomic Number Chemistry For Non Majors

Atomic Number Mass Number Definition Facts Videos Calculations With Examples And Faqs
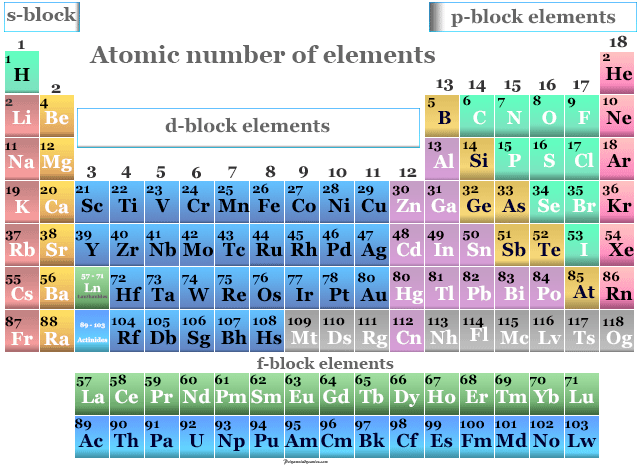
Comments
Post a Comment